What ion rushes into the neuron and causes it to become stimulated?
Learning Objectives
- Describe the components of the membrane that establish the resting membrane potential
- Describe the changes that occur to the membrane that result in the action potential
The functions of the nervous organisation—awareness, integration, and response—depend on the functions of the neurons underlying these pathways. To understand how neurons are able to communicate, it is necessary to draw the function of anexcitable membrane in generating these signals. The basis of this communication is the activity potential, which demonstrates how changes in the membrane can establish a indicate. Looking at the way these signals work in more variable circumstances involves a look at graded potentials, which will be covered in the next section.
Electrically Active Cell Membranes
Well-nigh cells in the body brand apply of charged particles, ions, to build up a charge across the prison cell membrane. Previously, this was shown to exist a function of how muscle cells work. For skeletal muscles to contract, based on excitation–contraction coupling, requires input from a neuron. Both of the cells make use of the cell membrane to regulate ion move between the extracellular fluid and cytosol.
As yous learned in the chapter on cells, the cell membrane is primarily responsible for regulating what can cross the membrane and what stays on simply one side. The cell membrane is a phospholipid bilayer, and so merely substances that can pass directly through the hydrophobic core can diffuse through unaided. Charged particles, which are hydrophilic past definition, cannot laissez passer through the jail cell membrane without aid (Figure 1). Transmembrane proteins, specifically channel proteins, make this possible. Several channels, also as specialized free energy dependent "ion-pumps," are necessary to generate a transmembrane potential and to generate an action potential. Of special interest is the carrier poly peptide referred to as the sodium/potassium pump that moves sodium ions (Na+) out of a cell and potassium ions (K+) into a cell, thus regulating ion concentration on both sides of the cell membrane.
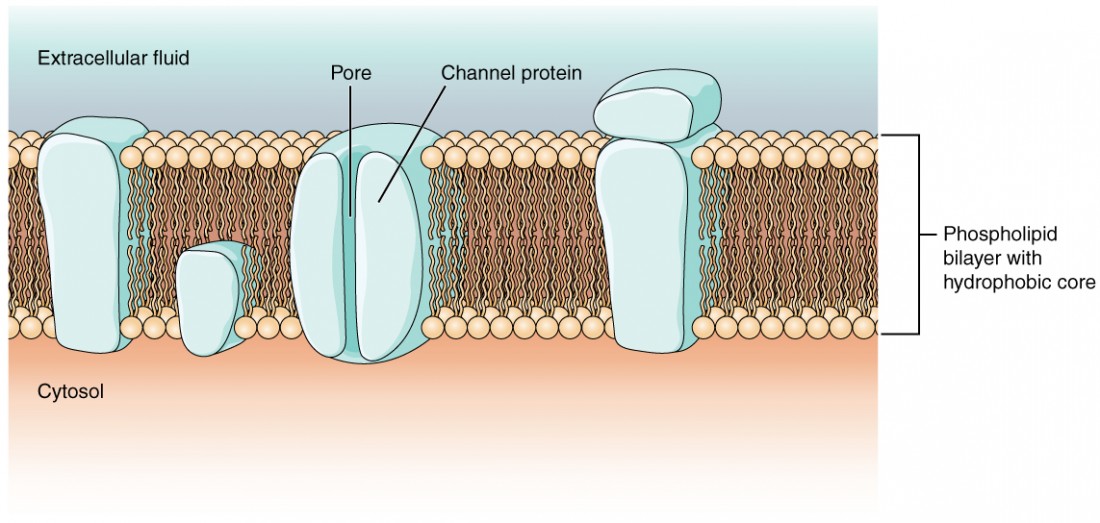
Figure i. Prison cell Membrane and Transmembrane Proteins The cell membrane is composed of a phospholipid bilayer and has many transmembrane proteins, including unlike types of aqueduct proteins that serve as ion channels.
The sodium/potassium pump requires free energy in the form of adenosine triphosphate (ATP), so it is as well referred to as an ATPase. Every bit was explained in the jail cell chapter, the concentration of Na+ is college outside the prison cell than inside, and the concentration of Thousand+ is higher inside the cell is higher than outside. That means that this pump is moving the ions against the concentration gradients for sodium and potassium, which is why it requires energy. In fact, the pump basically maintains those concentration gradients.
Ion channels are pores that permit specific charged particles to cantankerous the membrane in response to an existing concentration gradient. Proteins are capable of spanning the cell membrane, including its hydrophobic core, and can interact with the charge of ions because of the varied properties of amino acids found within specific domains or regions of the protein channel. Hydrophobic amino acids are found in the domains that are apposed to the hydrocarbon tails of the phospholipids. Hydrophilic amino acids are exposed to the fluid environments of the extracellular fluid and cytosol. Additionally, the ions will interact with the hydrophilic amino acids, which will exist selective for the charge of the ion. Channels for cations (positive ions) will take negatively charged side chains in the pore. Channels for anions (negative ions) will have positively charged side bondage in the pore. This is chosenelectrochemical exclusion, meaning that the channel pore is charge-specific.
Ions can also be specified by the diameter of the pore. The distance between the amino acids volition exist specific for the diameter of the ion when it dissociates from the water molecules surrounding it. Because of the surrounding water molecules, larger pores are not ideal for smaller ions because the water molecules volition interact, by hydrogen bonds, more readily than the amino acid side chains. This is calledsize exclusion. Some ion channels are selective for charge but not necessarily for size, and thus are chosen anonspecific channel. These nonspecific channels allow cations—particularly Na+, K+, and Catwo+—to cross the membrane, just exclude anions.
Ion channels do not always freely allow ions to diffuse beyond the membrane. They are opened by certain events, meaning the channels aregated. Then some other way that channels can be categorized is on the ground of how they are gated. Although these classes of ion channels are found primarily in cells of nervous or muscular tissue, they also can exist constitute in cells of epithelial and connective tissues.
Aligand-gated channel opens because a signaling molecule, a ligand, binds to the extracellular region of the channel. This type of channel is as well known equally anionotropic receptor because when the ligand, known as a neurotransmitter in the nervous system, binds to the protein, ions cross the membrane irresolute its accuse (Figure ii).

Figure 2. Ligand-Gated Channels When the ligand, in this case the neurotransmitter acetylcholine, binds to a specific location on the extracellular surface of the channel protein, the pore opens to allow select ions through. The ions, in this example, are cations of sodium, calcium, and potassium.
Amechanically gated channel opens because of a physical distortion of the cell membrane. Many channels associated with the sense of bear on (somatosensation) are mechanically gated. For instance, every bit force per unit area is applied to the peel, these channels open and let ions to enter the cell. Like to this type of channel would exist the aqueduct that opens on the basis of temperature changes, as in testing the water in the shower (Figure 3).
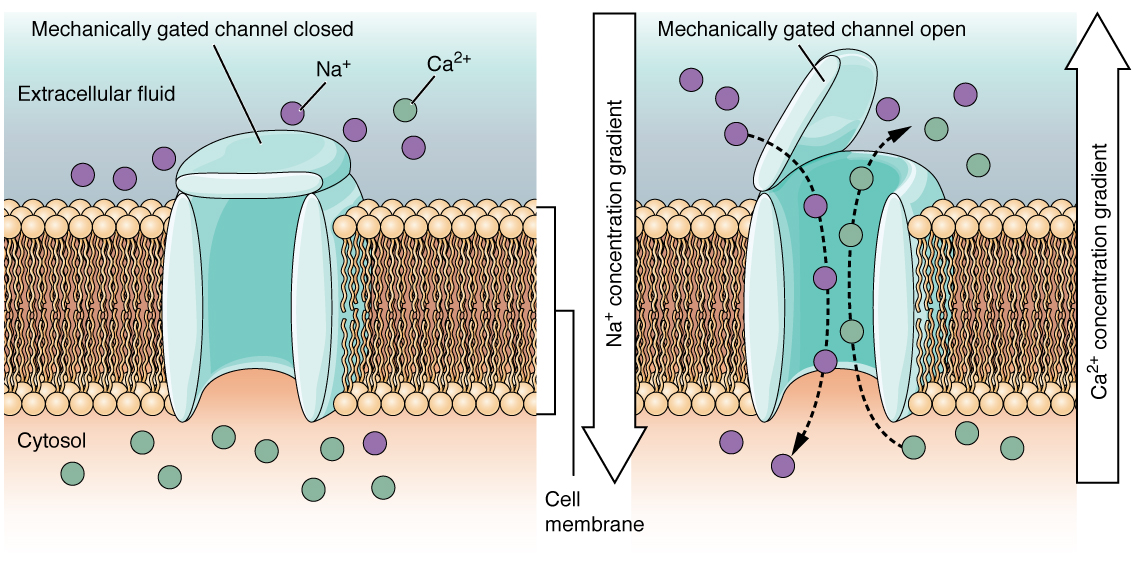
Figure 3. Mechanically Gated Channels When a mechanical alter occurs in the surrounding tissue, such equally pressure or touch, the aqueduct is physically opened. Thermoreceptors work on a similar principle. When the local tissue temperature changes, the protein reacts past physically opening the channel.
Avoltage-gated channel is a channel that responds to changes in the electrical properties of the membrane in which information technology is embedded. Normally, the inner portion of the membrane is at a negative voltage. When that voltage becomes less negative, the channel begins to permit ions to cross the membrane (Effigy 4).
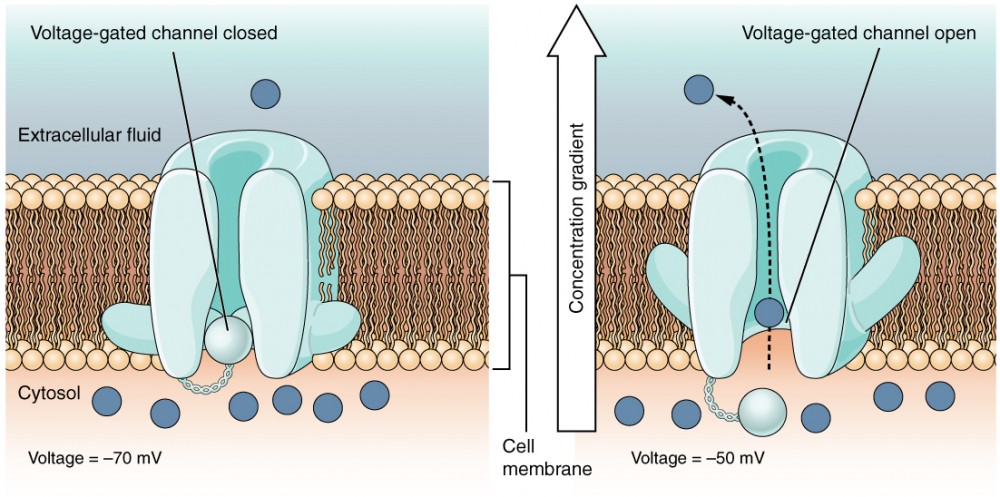
Figure 4. Voltage-Gated Channels Voltage-gated channels open when the transmembrane voltage changes around them. Amino acids in the structure of the protein are sensitive to accuse and cause the pore to open to the selected ion.
Aleakage channel is randomly gated, meaning that information technology opens and closes at random, hence the reference to leaking. There is no bodily effect that opens the channel; instead, information technology has an intrinsic rate of switching between the open and airtight states. Leakage channels contribute to the resting transmembrane voltage of the excitable membrane (Figure v).
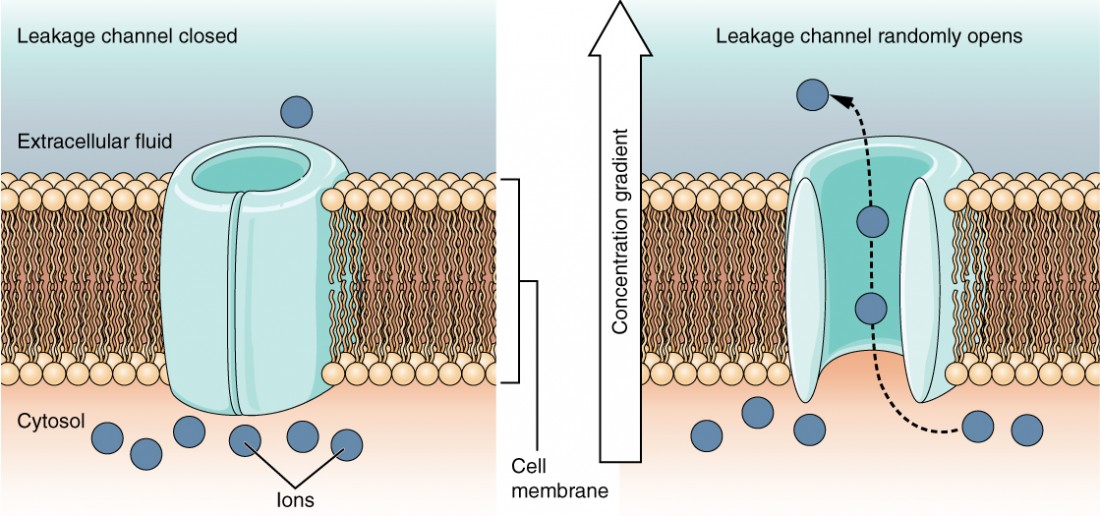
Figure 5. Leakage Channels In certain situations, ions need to move across the membrane randomly. The particular electric backdrop of sure cells are modified by the presence of this type of channel.
The Membrane Potential
The electrical state of the cell membrane tin can take several variations. These are all variations in themembrane potential. A potential is a distribution of charge across the cell membrane, measured in millivolts (mV). The standard is to compare the inside of the cell relative to the outside, so the membrane potential is a value representing the accuse on the intracellular side of the membrane based on the outside existence zero, relatively speaking (Figure 6).
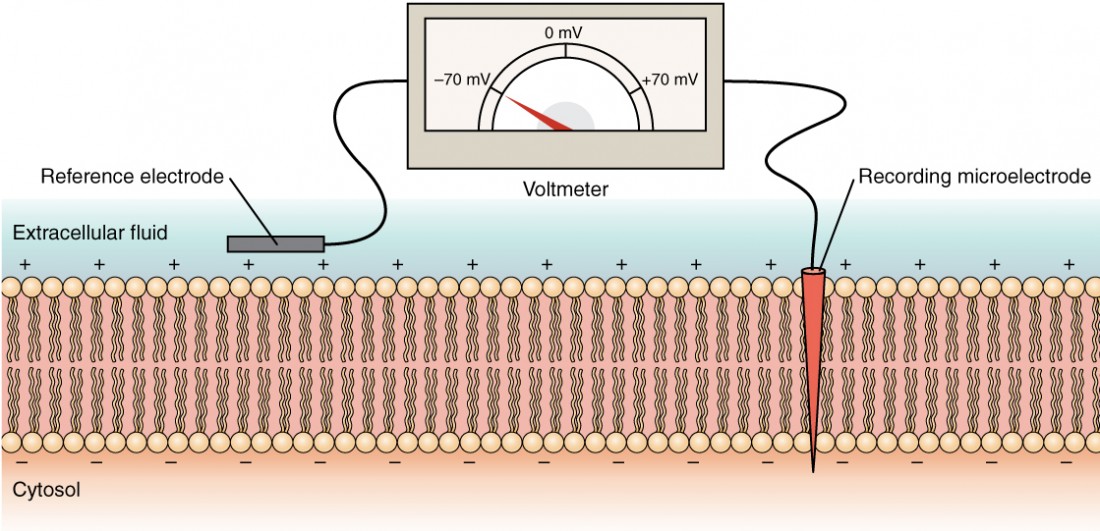
Figure 6. Measuring Accuse across a Membrane with a Voltmeter A recording electrode is inserted into the jail cell and a reference electrode is exterior the jail cell. By comparing the charge measured by these two electrodes, the transmembrane voltage is determined. Information technology is conventional to express that value for the cytosol relative to the outside.
The concentration of ions in extracellular and intracellular fluids is largely balanced, with a internet neutral charge. However, a slight divergence in charge occurs right at the membrane surface, both internally and externally. It is the divergence in this very limited region that has all the power in neurons (and muscle cells) to generate electrical signals, including activeness potentials.
Before these electrical signals tin be described, the resting state of the membrane must exist explained. When the cell is at rest, and the ion channels are closed (except for leakage channels which randomly open), ions are distributed across the membrane in a very predictable way. The concentration of Na+ outside the prison cell is 10 times greater than the concentration inside. Also, the concentration of K+ within the jail cell is greater than outside. The cytosol contains a high concentration of anions, in the grade of phosphate ions and negatively charged proteins. Large anions are a component of the inner cell membrane, including specialized phospholipids and proteins associated with the inner leaflet of the membrane (leaflet is a term used for one side of the lipid bilayer membrane). The negative charge is localized in the big anions.
With the ions distributed across the membrane at these concentrations, the difference in charge is measured at −70 mV, the value described as theresting membrane potential. The exact value measured for the resting membrane potential varies betwixt cells, but −70 mV is well-nigh usually used as this value. This voltage would actually be much lower except for the contributions of some of import proteins in the membrane. Leakage channels permit Na+ to slowly move into the cell or One thousand+ to slowly move out, and the Na+/K+ pump restores them. This may appear to be a waste of energy, but each has a role in maintaining the membrane potential.
The Action Potential
Resting membrane potential describes the steady state of the cell, which is a dynamic process that is counterbalanced by ion leakage and ion pumping. Without whatsoever outside influence, it will not change. To get an electrical betoken started, the membrane potential has to modify.
This starts with a channel opening for Na+ in the membrane. Because the concentration of Na+ is college exterior the cell than within the prison cell by a factor of 10, ions will blitz into the jail cell that are driven largely by the concentration gradient. Because sodium is a positively charged ion, it will change the relative voltage immediately inside the cell relative to immediately outside. The resting potential is the state of the membrane at a voltage of −lxx mV, and then the sodium cation inbound the cell will cause it to become less negative. This is known asdepolarization, meaning the membrane potential moves toward nada.
The concentration gradient for Na+ is so strong that it will continue to enter the prison cell even later on the membrane potential has become zero, and so that the voltage immediately around the pore begins to become positive. The electrical gradient also plays a role, as negative proteins beneath the membrane concenter the sodium ion. The membrane potential will reach +thirty mV past the time sodium has entered the cell.
Equally the membrane potential reaches +30 mV, other voltage-gated channels are opening in the membrane. These channels are specific for the potassium ion. A concentration slope acts on K+, every bit well. Every bit K+ starts to get out the cell, taking a positive accuse with it, the membrane potential begins to move back toward its resting voltage. This is calledrepolarization, pregnant that the membrane voltage moves back toward the −70 mV value of the resting membrane potential.
Repolarization returns the membrane potential to the −70 mV value that indicates the resting potential, but it actually overshoots that value. Potassium ions reach equilibrium when the membrane voltage is below −70 mV, so a period of hyperpolarization occurs while the One thousand+ channels are open. Those M+ channels are slightly delayed in closing, bookkeeping for this short overshoot.
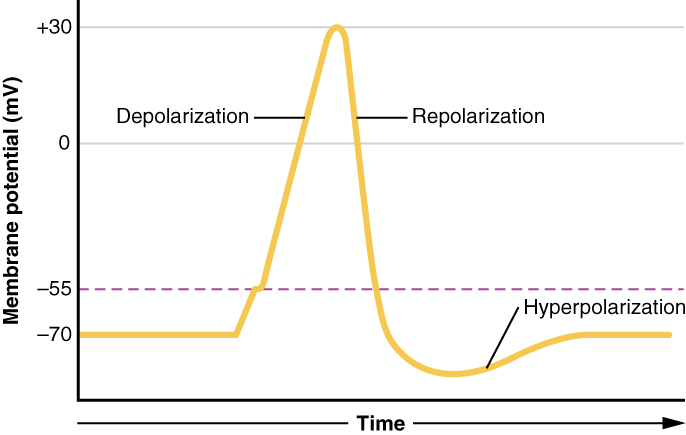
Figure 7. Graph of Action Potential Plotting voltage measured beyond the cell membrane against fourth dimension, the action potential begins with depolarization, followed past repolarization, which goes by the resting potential into hyperpolarization, and finally the membrane returns to balance.
What has been described here is the action potential, which is presented as a graph of voltage over time in Effigy 7. It is the electrical signal that nervous tissue generates for communication. The alter in the membrane voltage from −70 mV at rest to +30 mV at the end of depolarization is a 100-mV alter. That tin can also be written as a 0.ane-V change.
To put that value in perspective, think almost a battery. An AA bombardment that you lot might detect in a television remote has a voltage of i.five 5, or a 9-V battery (the rectangular battery with 2 posts on one stop) is, obviously, 9 Five. The modify seen in the activity potential is one or ii orders of magnitude less than the charge in these batteries. In fact, the membrane potential can be described as a battery. A accuse is stored across the membrane that tin can be released under the correct atmospheric condition. A battery in your remote has stored a charge that is "released" when you push button a button.
What happens across the membrane of an electrically active cell is a dynamic procedure that is hard to visualize with static images or through text descriptions. View this animation to learn more than about this process.
What is the difference between the driving force for Na+ and K+? And what is like about the motion of these two ions?
The question is, at present, what initiates the action potential? The description above conveniently glosses over that indicate. But it is vital to understanding what is happening. The membrane potential volition stay at the resting voltage until something changes. The description to a higher place simply says that a Na+ aqueduct opens. Now, to say "a aqueduct opens" does not mean that one individual transmembrane protein changes. Instead, it means that 1 kind of channel opens. There are a few different types of channels that allow Na+ to cross the membrane. A ligand-gated Na+ channel will open when a neurotransmitter binds to information technology and a mechanically gated Na+ channel will open when a physical stimulus affects a sensory receptor (similar force per unit area practical to the skin compresses a touch receptor). Whether it is a neurotransmitter binding to its receptor protein or a sensory stimulus activating a sensory receptor jail cell, some stimulus gets the process started. Sodium starts to enter the prison cell and the membrane becomes less negative.
A 3rd type of channel that is an important part of depolarization in the activeness potential is the voltage-gated Na+ channel. The channels that get-go depolarizing the membrane because of a stimulus aid the cell to depolarize from −70 mV to −55 mV. One time the membrane reaches that voltage, the voltage-gated Na+ channels open. This is what is known equally the threshold. Whatsoever depolarization that does not change the membrane potential to −55 mV or higher will not reach threshold and thus will not result in an action potential. Besides, any stimulus that depolarizes the membrane to −55 mV or beyond volition cause a large number of channels to open and an action potential will be initiated.
Because of the threshold, the action potential can be likened to a digital event—it either happens or it does not. If the threshold is not reached, and so no action potential occurs. If depolarization reaches −55 mV, then the activeness potential continues and runs all the way to +thirty mV, at which K+ causes repolarization, including the hyperpolarizing overshoot. Also, those changes are the same for every action potential, which means that once the threshold is reached, the exact same matter happens. A stronger stimulus, which might depolarize the membrane well past threshold, will non make a "bigger" action potential. Activeness potentials are "all or none." Either the membrane reaches the threshold and everything occurs as described in a higher place, or the membrane does not attain the threshold and zero else happens. All activeness potentials peak at the aforementioned voltage (+thirty mV), so ane activeness potential is not bigger than another. Stronger stimuli will initiate multiple activity potentials more quickly, but the private signals are non bigger. Thus, for example, you will not feel a greater sensation of pain, or have a stronger muscle wrinkle, because of the size of the activeness potential because they are not different sizes.
As nosotros take seen, the depolarization and repolarization of an action potential are dependent on 2 types of channels (the voltage-gated Na+ aqueduct and the voltage-gated G+ aqueduct). The voltage-gated Na+ aqueduct actually has two gates. One is theactivation gate, which opens when the membrane potential crosses −55 mV. The other gate is theinactivation gate, which closes after a specific menstruum of time—on the order of a fraction of a millisecond. When a prison cell is at rest, the activation gate is closed and the inactivation gate is open. However, when the threshold is reached, the activation gate opens, assuasive Na+ to rush into the jail cell. Timed with the pinnacle of depolarization, the inactivation gate closes. During repolarization, no more sodium tin can enter the cell. When the membrane potential passes −55 mV once again, the activation gate closes. After that, the inactivation gate re-opens, making the channel ready to start the whole process over once again.
The voltage-gated K+ aqueduct has only one gate, which is sensitive to a membrane voltage of −50 mV. However, it does not open up as speedily as the voltage-gated Na+ aqueduct does. Information technology might have a fraction of a millisecond for the channel to open once that voltage has been reached. The timing of this coincides exactly with when the Na+ menses peaks, so voltage-gated Thousand+ channels open just as the voltage-gated Na+ channels are being inactivated. Equally the membrane potential repolarizes and the voltage passes −50 mV again, the channel closes—once again, with a picayune delay. Potassium continues to exit the prison cell for a short while and the membrane potential becomes more negative, resulting in the hyperpolarizing overshoot. And then the aqueduct closes again and the membrane tin return to the resting potential considering of the ongoing action of the non-gated channels and the Na+/K+ pump.

Figure viii.Stages of an Action Potential
All of this takes place within approximately 2 milliseconds (Figure 8). While an activeness potential is in progress, another one cannot be initiated. That event is referred to as therefractory period. At that place are two phases of the refractory menstruation: theabsolute refractory period and therelative refractory period. During the absolute phase, another action potential will not beginning. This is because of the inactivation gate of the voltage-gated Na+ channel. Once that channel is back to its resting conformation (less than −55 mV), a new activeness potential could be started, but only by a stronger stimulus than the 1 that initiated the electric current activity potential. This is considering of the menses of Thou+ out of the prison cell. Because that ion is rushing out, whatever Na+ that tries to enter will not depolarize the cell, but will only go on the cell from hyperpolarizing.
Plotting voltage measured across the cell membrane against time (as shown in Figure viii), the events of the action potential tin exist related to specific changes in the membrane voltage.
- At rest, the membrane voltage is −70 mV.
- The membrane begins to depolarize when an external stimulus is applied.
- The membrane voltage begins a rapid rise toward +xxx mV.
- The membrane voltage starts to return to a negative value.
- Repolarization continues by the resting membrane voltage, resulting in hyperpolarization.
- The membrane voltage returns to the resting value shortly after hyperpolarization.
Propagation of the Action Potential
The action potential is initiated at the beginning of the axon, at what is called the initial segment. There is a high density of voltage-gated Na+ channels then that rapid depolarization tin take place here. Going down the length of the axon, the activeness potential is propagated because more than voltage-gated Na+ channels are opened as the depolarization spreads. This spreading occurs considering Na+ enters through the channel and moves forth the inside of the cell membrane. As the Na+ moves, or flows, a short distance forth the cell membrane, its positive charge depolarizes a little more of the cell membrane. As that depolarization spreads, new voltage-gated Na+ channels open and more ions rush into the cell, spreading the depolarization a petty farther.
Because voltage-gated Na+ channels are inactivated at the pinnacle of the depolarization, they cannot be opened again for a brief time—the accented refractory catamenia. Because of this, depolarization spreading back toward previously opened channels has no effect. The action potential must propagate toward the axon terminals; as a upshot, the polarity of the neuron is maintained, every bit mentioned above.
Propagation, equally described above, applies to unmyelinated axons. When myelination is present, the activity potential propagates differently. Sodium ions that enter the jail cell at the initial segment outset to spread along the length of the axon segment, but there are no voltage-gated Na+ channels until the first node of Ranvier. Because there is not constant opening of these channels along the axon segment, the depolarization spreads at an optimal speed. The distance between nodes is the optimal distance to proceed the membrane yet depolarized above threshold at the next node. As Na+ spreads along the within of the membrane of the axon segment, the accuse starts to dissipate. If the node were whatsoever farther down the axon, that depolarization would have fallen off too much for voltage-gated Na+ channels to be activated at the adjacent node of Ranvier. If the nodes were whatever closer together, the speed of propagation would be slower.
Propagation along an unmyelinated axon is referred to ascontinuous conduction; forth the length of a myelinated axon, information technology issaltatory conduction. Continuous conduction is boring because there are ever voltage-gated Na+ channels opening, and more and more Na+ is rushing into the prison cell. Saltatory conduction is faster considering the action potential basically jumps from one node to the next (saltare = "to leap"), and the new influx of Na+ renews the depolarized membrane. Along with the myelination of the axon, the diameter of the axon can influence the speed of conduction. Much every bit water runs faster in a wide river than in a narrow creek, Na+-based depolarization spreads faster down a wide axon than down a narrow one. This concept is known equallyresistance and is generally true for electrical wires or plumbing, just as it is truthful for axons, although the specific conditions are different at the scales of electrons or ions versus water in a river.
Homeostatic Imbalances: Potassium Concentration
Glial cells, especially astrocytes, are responsible for maintaining the chemical environment of the CNS tissue. The concentrations of ions in the extracellular fluid are the basis for how the membrane potential is established and changes in electrochemical signaling. If the balance of ions is upset, drastic outcomes are possible.
Unremarkably the concentration of K+ is college inside the neuron than outside. Later on the repolarizing phase of the action potential, Thou+ leakage channels and the Na+/Yard+ pump ensure that the ions render to their original locations. Following a stroke or other ischemic upshot, extracellular K+ levels are elevated. The astrocytes in the area are equipped to clear excess K+ to aid the pump. Only when the level is far out of residue, the furnishings can be irreversible.
Astrocytes can become reactive in cases such as these, which impairs their power to maintain the local chemical environs. The glial cells overstate and their processes cracking. They lose their K+ buffering ability and the office of the pump is affected, or fifty-fifty reversed. If a Na+ gradient breaks downwardly, this has a more important issue than interrupting the action potential. Glucose transport into cells is coupled with Na+ co-transport. When that is lost, the cell cannot get the free energy it needs. In the central nervous system, carbohydrate metabolism is the only means of producing ATP. Elsewhere in the torso, cells rely on carbohydrates, lipids, or amino acids to ability mitochondrial ATP production. But the CNS does not store lipids in adipocytes (fat cells) equally an energy reserve. The lipids in the CNS are in the jail cell membranes of neurons and glial cells, notably as an integral component of myelin. Proteins in the CNS are crucial to neuronal role, in roles such as channels for electrical signaling or as role of the cytoskeleton. Those macromolecules are not used to ability mitochondrial ATP production in neurons.
Visit this site to see a virtual neurophysiology lab, and to observe electrophysiological processes in the nervous organisation, where scientists directly measure the electric signals produced past neurons. Often, the activeness potentials occur so rapidly that watching a screen to see them occur is not helpful. A speaker is powered by the signals recorded from a neuron and it "pops" each time the neuron fires an action potential. These action potentials are firing so fast that it sounds like static on the radio. Electrophysiologists tin recognize the patterns within that static to sympathise what is happening. Why is the leech model used for measuring the electric activity of neurons instead of using humans?
Source: https://courses.lumenlearning.com/cuny-csi-ap-1/chapter/the-action-potential/
0 Response to "What ion rushes into the neuron and causes it to become stimulated?"
Post a Comment